Overview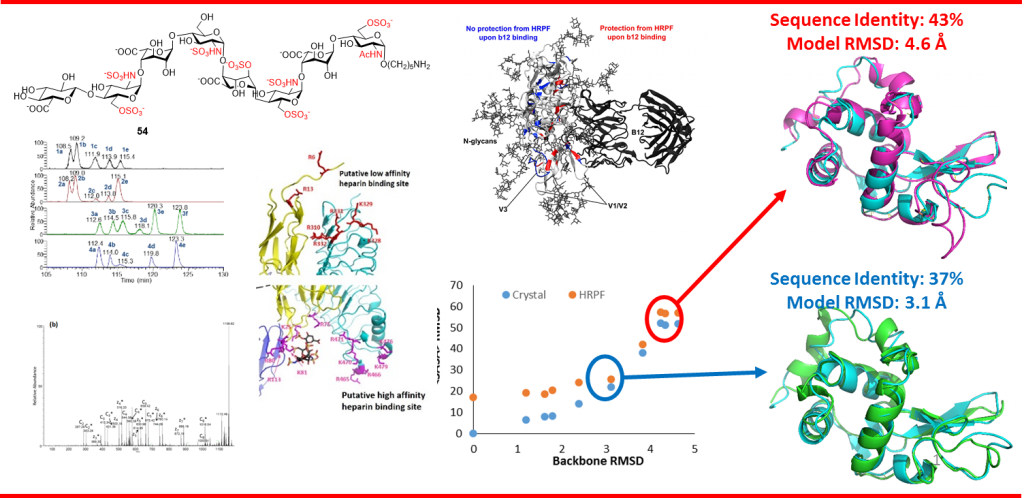
Dr. Sharp’s group is active in the development and application of new mass spectrometry-based technologies for studying the structure-function relationships of proteins and carbohydrates of biomedical interest. Current applications focus on the study of interactions between neutralizing antibodies and the glycoprotein coat of HIV; characterization of chemokines and the structural factors mediating their oligomerization and function; and the identification of glycosaminoglycan structures with potential biomedical applications for a wide variety of fields including anti-microbial agents, anti-cancer therapies, and anti-inflammatory therapies.
Recent Selected Publications
Abolhasani Khaje N., Eletsky A., Biehn S., Mobley C., Rogals M., Kim Y., Mishra S., Doerksen R., Lindert S., Prestegard J., and Sharp J.S. Validated Determination of NRG1 Ig-like Domain Structure by Mass Spectrometry Coupled with Computational Modeling (preprint)
High resolution hydroxyl radical protein footprinting (HR-HRPF) is a mass spectrometry-based method that measures the solvent exposure of multiple amino acids in a single experiment, offering constraints for experimentally-informed computational modeling. HR-HRPF-based modeling has previously been used to accurately model the structure of proteins of known structure, but the technique has never been used to determine the structure of a protein of unknown structure leaving questions of unintentional bias and applicability to unknown structures unresolved. Here, we present the use of HR-HRPF-based modeling to determine the structure of the Ig-like domain of NRG1, a protein with no close homolog of known structure. Independent determination of the protein structure by both HR-HRPF-based modeling and heteronuclear NMR was carried out, with results compared only after both processes were complete. The HR-HRPF-based model was highly similar to the lowest energy NMR model, with a backbone RMSD of 1.6 Å. To our knowledge, this is the first use of HR-HRPF-based modeling to determine a previously uncharacterized protein structure.
Mitra S., Talukdar K., Prasad P., Misra S., Khan S., Sharp J., Jurss J., and Chakraborty S. Rational Design of a Cu Chelator that Mitigates Cu‐Induced ROS Production by Amyloid Beta. ChemBioChem. 2021 Dec 1.
Alzheimer’s disease severely perturbs the transition metal homeostasis in the brain leading to the accumulation of excess metals in extracellular and intraneuronal locations. The amyloid beta protein binds these transition metals, ultimately causing severe oxidative stress in the brain. Metal chelation therapy is an approach to sequester metals from amyloid beta and relieve the oxidative stress. Here we have designed a mixed N/O donor Cu chelator inspired by the proposed ligand set of Cu in amyloid beta. We demonstrate that the chelator effectively removes Cu from amyloid beta and suppresses ROS production by redox silencing and radical scavenging both in vitro and in cellulo. The impact of ROS on the extent of oxidation of the different aggregated forms of the peptide is studied by mass spectrometry, which, along with other ROS assays, shows that the oligomers are pro‐oxidants in nature. The aliphatic Leu34, which was previously unobserved, has been identified as a new oxidation site.
Akbar S., Phillips K.E., Misra S.K., Sharp J.S. and Stevens D.C. Differential response to prey quorum signals indicates predatory specialization of myxobacteria and ability to predate Pseudomonas aeruginosa. Environ Microbiol. 2021 Oct 21.
Multiomic analysis of transcriptional and metabolic responses from the predatory myxobacteria Myxococcus xanthus and Cystobacter ferrugineus exposed to prey signalling molecules of the acylhomoserine lactone and quinolone quorum signalling classes provided insight into predatory specialization. Acylhomoserine lactone quorum signals elicited a general response from both myxobacteria. We suggest that this is likely due to the generalist predator lifestyles of myxobacteria and ubiquity of acylhomoserine lactone signals. We also provide data that indicates the core homoserine lactone moiety included in all acylhomoserine lactone scaffolds to be sufficient to induce this general response. Comparing both myxobacteria, unique transcriptional and metabolic responses were observed from Cystobacter ferrugineus exposed to the quinolone signal 2-heptylquinolin-4(1H)-one (HHQ) natively produced by Pseudomonas aeruginosa. We suggest that this unique response and ability to metabolize quinolone signals contribute to the superior predation of P. aeruginosa observed from C. ferrugineus. These results further demonstrate myxobacterial eavesdropping on prey signalling molecules and provide insight into how responses to exogenous signals might correlate with prey range of myxobacteria.

